Is sodium cumene sulfonate safe?
Sodium cumene sulfonate, a chemical compound commonly used in various industrial and commercial applications, has raised questions about its safety and potential impacts on human health and the environment. As with any chemical substance, understanding its safety profile is of paramount importance. In this article, we delve into the characteristics, applications, and safety considerations surrounding sodium cumene sulfonate.
Chemical Overview
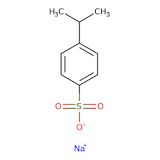
Sodium cumene sulfonate, chemically represented as C9H11SO3Na, is derived from cumene, a hydrocarbon compound, and sulfonic acid. It is soluble in water and often appears as a colorless to pale yellow liquid or solid. The compound is valued for its surfactant properties, which allow it to reduce the surface tension of liquids, enhancing their ability to mix and interact with other substances. This property makes sodium cumene sulfonate a key ingredient in various industrial formulations, including detergents, cleaners, and drilling fluids.
Applications and Industrial Uses
Sodium cumene sulfonate finds application in a range of industrial sectors due to its versatile properties. In the oil and gas industry, it is used as a component in drilling muds and fluids to improve fluidity and prevent clay swelling. Its surfactant properties contribute to the dispersion of drilling cuttings, aiding in the efficient extraction of oil and gas.
The compound also plays a role in the manufacturing of consumer products. In household cleaners and detergents, sodium cumene sulfonate serves as a surfactant, assisting in the removal of dirt and grease from surfaces. Additionally, it is utilized in the formulation of personal care products like shampoos, where it helps distribute and solubilize ingredients.
Safety Considerations
The safety of sodium cumene sulfonate is a subject of scrutiny and research, especially concerning its potential impacts on human health and the environment. When handled and used appropriately, the compound is generally considered safe in many industrial and consumer applications. However, like any chemical substance, there are important considerations to take into account:
Exposure and Inhalation: Sodium cumene sulfonate is primarily used in industrial settings, where proper ventilation and personal protective equipment are employed to minimize exposure risks. Prolonged or intense inhalation of the compound's aerosolized form can potentially irritate the respiratory system.
Skin Contact: Direct skin contact with concentrated forms of sodium cumene sulfonate may lead to skin irritation. Adherence to safety protocols, such as wearing protective clothing and gloves, is essential when handling the compound.
Environmental Impact: Sodium cumene sulfonate's use in drilling fluids has raised concerns about its potential environmental impact. Spills or improper disposal of drilling fluids containing the compound could lead to soil and water contamination. It is imperative to follow best practices for handling and disposing of such fluids to minimize environmental harm.
Biodegradability: Sodium cumene sulfonate is considered readily biodegradable, which means that under appropriate conditions, it can be broken down by natural processes, reducing its persistence in the environment.
Conclusion
Sodium cumene sulfonate is a chemical compound with diverse applications in industries ranging from oil and gas to consumer products. Its surfactant properties make it valuable for enhancing fluid interactions and cleaning capabilities. While the compound is generally safe when used as directed and with proper precautions, it is important to remain vigilant about potential exposure risks in industrial settings. Additionally, responsible handling and disposal practices are crucial to prevent environmental contamination. As our understanding of chemical compounds and their effects continues to evolve, ongoing research and adherence to safety guidelines will ensure that sodium cumene sulfonate can be used effectively while minimizing potential hazards.
206
0
0


Comments
All Comments (0)